My Summary Assessment of Immunoglobulin IgG IVIG for CIDP 2011 until 2020: Intravenous Infusions (IVIG) or Sub-cutaneous (SCIG or Sub-Q) therapy for Chronic Inflammatory Demyelinating Polyneuropathy
As at January 2019. I have had seven IgG Immunoglobulin campaigns since March 2011 but I only started keeping full log of its effect from July 2013. I treated the March 2011 IVIG campaign as a standard therapy that was going to cure me of CIDP and was somewhat taken back when two years later I required more treatment with a campaign starting July 2013. I have kept some monthly and more recently daily records of how I feel on each of the symptoms caused by my CIDP since that campaign. I have tried to be as scientific as one can be trying to measure real happenings that day - such as whether my leg actually fully cramped. It is qualitative but I have found that my indices follow reasonably closely with parallel medical nerve conduction studies ("NCS" every six months). IgG has not cured me of CIDP (not even resulted in a short remission) , but I feel much better on IgG than when I am off it. Indeed I hate to think where I would be now with my CIDP if I had not had access to this blood product! IgG is delivered intravenously (IVIG) or subcutaneously (SCIG or Sub-q). I have received various brands of IVIG (Gamunex, Privigen, IGIVnex, Panzyga) as well as SCIG / Sub-Q (Hizentra, Cuvitru) and have recorded the dates. Details of each campaign are given on other pages but the following is my summary assessment of the IgG infusion campaign impacts I have experienced -
Summary IgG IVIG & SCIG Effectiveness 2011-2020
» March 2011 my first campaign of IVIG infusions commenced lasting five months at full dose (booster plus 70g taken monthly). I did not keep notes of that campaign but recall that it took about three months for me to feel the benefits, and only after completion when CIDP symptoms returned did I realize the high significance of these benefits.
» IVIG was quite effective early on (2013) in my CIDP history but less so lately (2015-2016) but overall extremely helpful in my CIDP management and daily life!
» Indeed the 2013-14 campaign was so effective that my recovery continued afterwards for seven months
» My (life threatening) breathing issue was averted in 2013 and IVIG is still undeniably in 2016 protecting me
» In the 2015 campaign, early encouraging signs were reversed, I theorize due to overdosing with three loading doses (Conductive studies found I had actually worsened during the campaign)
» It seems from the detailed analysis that the older the symptom the more effective IVIG is in controlling it: For me very notable mitigation of "electrical vibrations", fasciculations, cramping, sea-sickness and breathing
» Indeed, the last campaign (2015) was completely ineffective against new upper body symptoms
» Symptom relief from my CIPD can take a day after first infusion (eg., fasciculations, vibration, cramping, pain reduction) to several months (eg., balance, motion, and breathing issues)
» Subcutaneously infused IG - Sub-Q or SCIG late 2015 - of Hizentra proved ineffective in my case although I would like a re-trial* as I believe extraneous circumstances (loading doses) intervened in my one trial
»The maintenance low-dose IVIG commenced march 2016 are adequate but the overall impact of low maintenance dose on my symptoms are less effective than the usual full dose - as of my 9th dose mid-October 2016 I am beginning to question its efficacy as my symptoms are barely tolerable and new upper body issues getting worse
» October 26, 2016 blood tests show I have elevated thyroid TSH and Pituitary FSH hormones**. Investigations continue Nov 14, 2016 and I hope this is not serious - I will find out more January 5, 2017
» December 6, 2016 the CIDP clinic says conductive studies indicate stability (since March 2016). This should be good news but for me represents a significant disconnect with the symptoms I live with every day. My IVIG maintenance therapy is to continue - I certainly hope I am wrong on the progression issue!.
» April 2, 2017 a year into the maintenance dose of 40 g IVIG every four weeks I feel my symptoms are stabilizing albeit at an uncomfortable level for daily living. However, the stability is comforting - confirmed July 19 by NCS!
» October 15, 2017 With pain now at "non-livable" level, I started experimenting with cannabidiol CBD cannabis as a potential CIDP pain killer but by November 2017 I felt no relief from the cannabidiol and abandoned the idea.
» November 28, 2017 My check-up, including Nerve Conduction Studies (NCS), indicates stability so it looks like I am ending the year 2017 in about the same condition as I started in January although perhaps more pain and progression in my my left arm.
» Since January 2018 I am participating in a year-long trial of Panzyga IVIG manufactured by Octapharma involving a higher standard dosage (70g per 4-weeks) than my current maintenance level (of 40g).
» June 2018 I began I trial of very high "pulse-dose" dexamethasone corticosteroid aimed at improving outcomes and suppressing the adverse effects of IVIG. The experiment was stopped after two months and proved highly counter-productive in my case.
» July 6, 2018 Half way through the Panzyga trial and NCS show neuromuscular stability. The astounding high 606 ml-hr infusion rate provides a significant advantage (contrary to current thinking that a slow infusion rate is better)
» November 23, 2018 My last Panzyga infusion. Based on my index I became quite variable but I believe this was due to the dexamethasone.
» December 07, 2018 - I end the year with NCS evaluation results indicating stability. Thankfully no progression but disappointingly no improvement either.
» December 21, 2018 : A retry of Sub-Q (my 2015 trial of Hizentra was abandoned) this time with Cuvitru.
» December 29, 2019. My year on a weekly 31g dose of sub-q Cuvitru has largely gone well. I had a brutal rash for a while and some swollen lymph nodes. The side-effect persisted for six months but this is now subsiding. I feel stable entering 2020 and will remain on Cuvitru.
» September 25, 2020: The Cuvitru rash that started August 1, 2019 did not go away (as I predicted) but rather is causing extreme discomfort (stinging pain, bruised skin and deep red patches).
» October 12, 2020: I have developed quite severe upper arm & shoulder pain recently suggesting that something may be going wrong in the last few months - rash worse, hair loss and new arm pain.
» October 22, 2020: On the advice of my doctors I stopped my weekly CUVITRU scig infusion due to arm pain and potential adverse reaction. My doctor will review the situation November 3, 2020.
» November 03, 2020: Doctors believe I am having an adverse "allergic reaction" to CUVITRU or IgG, and as a result I am off all IgG for at least one month. Instead I will transition to daily 25mg dose of Prednisone pills for November 2020 and have another CIDP clinic review December 8, 2020 at which point to decide on future treatment.
» December 08, 2020: My doctor is uncomfortable putting me back on IgG due to the adverse reaction issue. Looks like my decade of IgG treatment for CIDP has ended early. An uncertain future looms entering 2021
» December 08, 2020: My journal continues at
> My CIDP Log after quitting IgG infusions from 2020.
> My CIDP since going solo from 2022
In 2018 I started a new CIDP Symptoms Index that excluded the "breathing" issue which resolved to normal in 2017. I added strength measurement components based on instrument records I have kept since 2015. The results are shown in the following chart -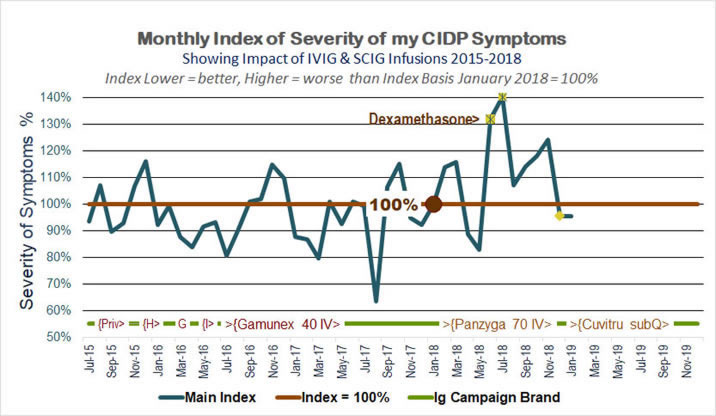
The earlier CIDP Symptoms Index is based on February 2015 = 100 and represents a daily log of a basket of my symptoms since 2013 that INCLUDES the major - if not dominating - breathing issue (since resolved by 2017), as shown below -

>> view larger CIDP-IVIG-impact-chart-summary-2017-11-28.pdf
You can also view some summary tables at my CIDP Experiments

>> view CIDP-IVIG-impact-chart-leg-hand-2013-2017.pdf
The above is my summary log chart and findings on my CIDP infusions but
the detailed impact of each IVIG campaign can be viewed at the following links -
* My usual IVIG dose is 72 g infused once per month or 1g per kg body weight per month.
** TSH = Thyroid Stimulating Hormone FSH = Follicle Stimulating Hormone I will see a endocrinologist November 14, 2016 to investigate why these two hormones are elevated. I am concerned that IVIG has caused this or alternatively that I have some kind of Autoimmune Polyendocrine Syndrome.
Key words My Summary Assessment of Immunoglobulin IVIG for CIDP 2016: Intravenous Infusions (IVIG) or Sub-cutaneous (Sub-Q): IVIG infusions for CIDP Chronic Inflammatory Demyelinating Polyneuropathy, IVIG Therapy for CIDP, CIDP IVIG Infusions, IVIG methods, IVIG procedure, CIDP example, IVIG Sample, IVIG CIDP quantity, IVIG prescription, drugs for CIDP, how to infuse IVIG for CIDP, treatment by IVIG for CIDP, how long does IVIG take to infuse for CIDP, CIDPlog chart Track IVIG impact Long term use of IVIG for CIDP Dose Intravenous Immunoglobulin IVIg Treatment for CIDP: CIDP dosage IVIG effectiveness, efficacy of IVIG in CIDP cases, IVIG comparison low dose versus high dose, trial outcome of IVIG infusion, IVIG therapy options low, medium high dose, est dose level, what is the optimal dose immunoglobulin for for CIDP, cidp worse with ivig high dose or low dose. immunoglobulin for for CIDP, cidp worse with ivig high dose or low dose immunoglobulin Igg or IVIG immunoglobulin IG
